This simple add-on to any Hologic 3D Mammography™ capable system enables quick 2D or 3D™ breast biopsies using the same imaging equipment as for mammography exams. Plus, the lateral arm upright biopsy accessory offers even more flexibility to access challenging lesions.
Stereotactic
Superior image quality 4
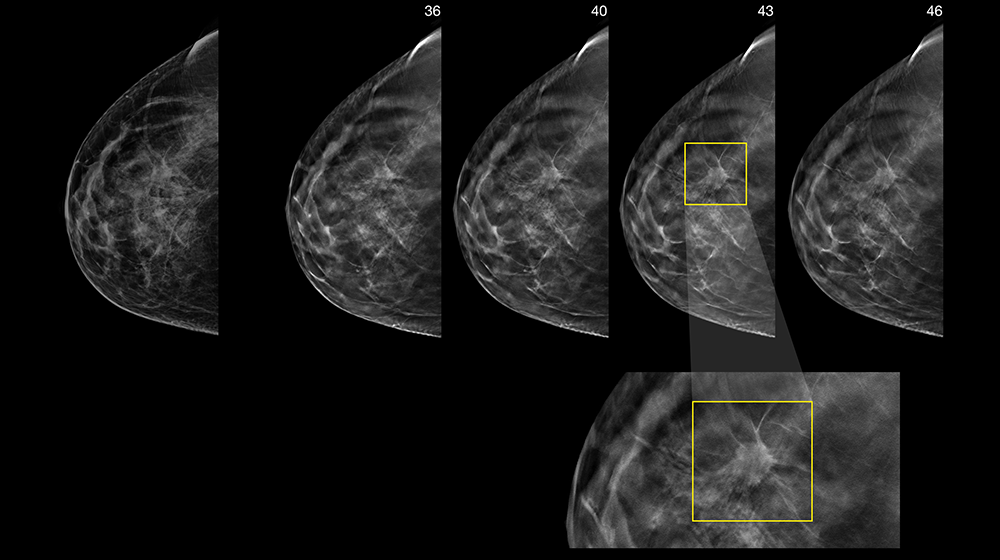
Pinpoint subtle lesions and faint calcifications—including those that may only be visible with tomosynthesis with the 3D™ biopsy option.
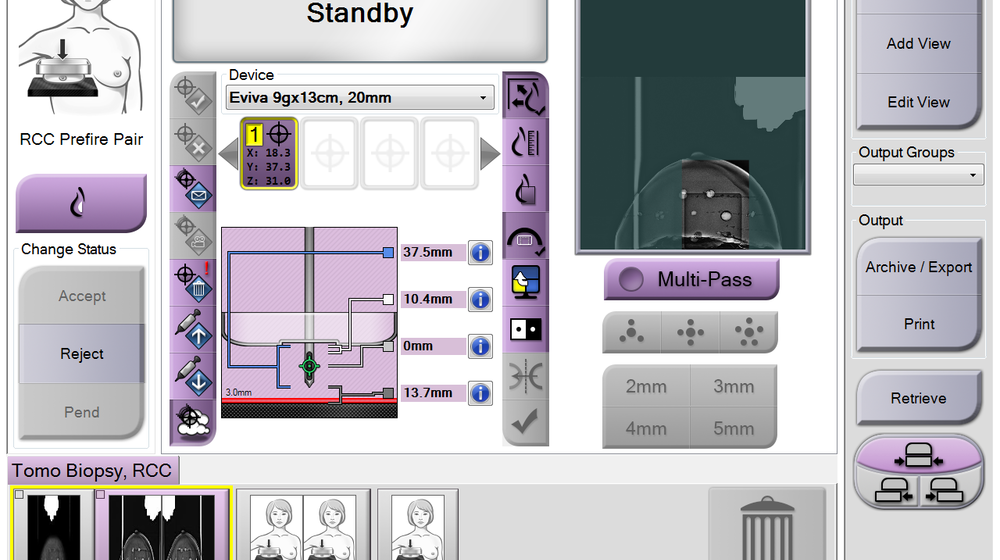
Efficient, fast, easy to learn and use. The system provides a simplified user interface to deliver a fast biopsy with fewer steps and a lower patient dose. 5

Transition from screening to interventional procedures to biopsy a wide spectrum of patients either seated or decubitus. Quickly and easily switch between standard and lateral needle approaches.
A large field of view for high-resolution stereotactic 2D or 3D™ images makes visualizing suspicious areas fast and simple.
Fast and easy to use 5
Intuitive user interface, fully integrated with the Selenia ® Dimensions ® and 3Dimensions™ systems, minimizes procedure steps and simplifies workflow with an average procedure time of 13 minutes.
Designed to remove the guesswork
Settings for all Hologic biopsy needle devices are pre-programmed, for quick setup and efficient operation, eliminating mental math with less chance for errors.
Access challenging lesions
Access challenging lesions with the optional lateral arm accessory. It installs quickly to present the needle parallel to the detector–especially valuable for thinly compressed breasts.
Affirm ® Lateral Arm Upright Biopsy Accessory
Confidently target challenging lesions and successfully biopsy thinly-compressed breasts. This versatile, easy-to-use device enables needle access parallel to the detector – from either the lateral left or lateral right position. Choose the optimal approach based on each patient’s anatomy and lesion location to accelerate and simplify biopsy procedures.
Flexible patient positioning
Positioning patients in constrained spaces is always the challenge. The Affirm lateral arm offers a number of options in biopsy rooms where decubitus positioning or rotating the C-arm may not be possible. Patients can often remain seated without sacrificing lesion accessibility.
Intuitive needle visualization
Experience improved 3D™ visualization of the biopsy needle in the breast with virtually no imaging artifacts as found in the standard approach. By presenting the needle parallel to the detector, it’s easier to see the needle aperture and more intuitive to make adjustments.
Tech Specs| Tech Specs | |
|---|---|
| Biopsy Volume | |
| Standard or Axillary Paddle | 5 cm x 5 cm x 10 cm |
| Wide Access Paddle | 6 cm x 7 cm x 10 cm |
| Compression Method | Motorized and Manual Compression |
| Compression Range | Up to 15 cm |
| Image Area | 18 cm x 24 cm |
| Breast Positioning Area | 24 cm x 29 cm |
| SID | 70 cm |
| Weight | < 7kg (< 15lb) |
| Breast Biopsy Guidance | |
| Needle Guidance | Cartesian Coordinate System Z-Axis has 10° tilt |
| Accuracy | +/-1 mm |
| Stereotactic Angle | +/-15° |
| C-Arm Positioning | +180° to -140°(stereo start locations) |
| Guidance Movements | X- and Y-Axes: Motorized Z-Axis: Manual |
| Range of Movement | 75 mm (width) x 70 mm (depth) x 160 mm (height) |
| Home Positions | Far Left, Far Right |
| Biopsy Field Illumination | Integrated LED |
| Biopsy Control Module | |
| Display Window | Touchscreen Color LCD 800 pixels x 600 pixels |
| Lesion Coordinate Display | Numeric, Cartesian |
| Display Mode | Target Guidance, Jog Screen, Select Targets |
| Acquisition Workstation | |
| Workflow Display | 1.2 MP Touchscreen Color or Standard Color LCD display |
| Image Display | 2 MP or 3 MP Medical Grade LCD DICOM Display |
| Biopsy Device Compatibility | |
| Needle Length | Up to 140mm |
| Biopsy Compression Paddles | |
| Standard Biopsy Paddle | |
| Compression Area | 14 cm x 18 cm |
| Biopsy Opening | 5.4 cm x 5.2 cm |
| Standard Biopsy Paddle | |
| Compression Area | 14 cm x 18 cm |
| Biopsy Opening | 7.4 cm x 6.2 cm |
| Axillary Biopsy Paddle | |
| Compression Area | 9.4 cm x 18 cm |
| Biopsy Opening | 5.4 cm x 5.2 cm |
| Environmental Conditions | |
| Operating Temperature | 20º to 30º C |
| Operating Relative Humidity | 20% to 80%, non-condensing |
| Storing Temperature | -10º to 40º C |
| Requirements | |
| Selenia® Dimensions® system diagnostic license and dynamic tube head motion license for biopsy | |
| Accessories | |
| Geometry Calibration Phantom | |
| Targeting Phantom | |
| Quality Assurance Needle | |
| Needle Guide Holder | |
| Affirm Biopsy License (single gantry) | |
| Tabletop Stand | |
| User Manual | |
| Service Manual | |
| Optional Components | |
| Lateral Arm | Enables needle access parallel to the detector from either the lateral left or right position |
| Biopsy Device Mounting | Standard adapters used on MultiCare ® Platinum and Digital StereoLoc ® II |
| Software Options | A rm 3DTM Biopsy License (single gantry) Additional A rm 3DTM Biopsy License (single gantry) Additional A rm Stereotactic Biopsy License (single gantry) |
| Environmental Conditions | |
| Operating Temperature | 20˚ to 30˚ C |
| Operating Relative Humidity | 20% to 80%, non-condensing |
| Storing Temperature | -10˚ to 40˚ C |



© 2022 Hologic. 3D, 3D Mammography, Affirm, ATEC, Brevera, Contura, CorLumina, Celero, Dimensions, Eviva, MammoSite, SecurMark, Selenia, Sertera, Trident, TriMark, 5-Day Targeted Therapy, Viera, The Science of Sure and associated logos are trademarks and/or registered trademarks of Hologic, Inc., and/or its subsidiaries in the United States and/or other countries. See all trademarks. Tumark and associated logos are trademarks and/or registered trademarks of Somatex Medical Technologies GmbH. Hologic is an authorized distributor of Tumark Professional X, Q, Flex and Vision biopsy site markers.
1 Compared to the MultiCare® Platinum System.
2 Preference survey results of 155 Eviva users – 2015.
3 Based on a survey of 165 patients post-procedure at 3 hospitals.
4 Compared to 2D.
5 Schrading S, Martine D, Dirrichs T, et al. “Digital Breast Tomosynthesis-guided Vacuum-assisted Breast biopsy: Initial Experiences and Comparison with Prone Stereotactic Vacuum-assisted Biopsy.” Radiology. 2015 274:3, 654-662 E-pub 2014 Nov 12.
6 Compared to standard approach.
7 Internal testing performed at Hologic and maintained in PLM, 2006, SecurMark, TriMark biopsy site markers.
9 Compared to Eviva device. 2017 data on file.
10 Internal testing performed at Hologic and maintained in PLM, 2007, Celero biopsy device.
11 Englander, B., 2013. An Evaluation of the Hologic® Celero® Vacuum Assisted Handheld Device for Ultrasound Guided Breast Biopsies, WP-00006 Rev.002 (09/13) US/International.
12 Kaplan, S. 2010. The Benefits of the Hologic Eviva Vacuum-Assisted Stereotactic Breast Biopsy Device, WP-00023 (4/11).
13 Data collected from 100 cases performed at various sites using the Sertera biopsy device.
14 2016 Kadence International survey of 200 healthcare professionals.
15 Based on Tumark® Data Collection Study, 3 clinicians at 3 hospitals for 71 marker placements, 2017.
16 Based on Tumark® Data Collection Study, 3 clinicians at 3 hospitals for 45 marker placements, 2017.
17 Shah C, Badiyan S, Ben Wilkinson J, Vicini F, Beitsch P, Keisch M, Arthur D, Lyden M. Treatment Efficacy with Accelerated Partial Breast Irradiation (APBI): Final Analysis of the American Society of Breast Surgeons MammoSite® Breast Brachytherapy Registry Trial. Ann Surg Oncol. 2013 Aug 22. [Epub ahead of print] PubMed PMID: 23975302.
18 King TA, Bolton JS, Kuske RR, et al. Long-term results of wide-field brachytherapy as the sole method of radiation therapy after segmental mastectomy for Tis1,2 breast cancer. Am J Surg. 2000; 180:299-304.
19 All clinical data used to support this 5-Day Targeted Therapy™ system claim was derived from use of the Mammosite® system.
20 Internal testing performed at Hologic and maintained in PLM 2018, Eviva biopsy device.
21 Internal testing performed at Hologic and maintained in PLM 2016, Sertera biopsy device.